The catalyst carrier can also affect the catalytic selectivity?
2024-08-02
chemical reactionto a large extentCatalyst-dependentAt present, the commonly used heterogeneous catalysts

chemical reactionto a large extentCatalyst-dependentAt present, the commonly used heterogeneous catalysts are based onInexpensive solid metal oxide supports supporting transition metal nanoparticles. The high surface to volume ratio of the support increases the utilization of the metal, such as a monatomic catalyst.Reducing the size of the noble metal affects the reactivity of the catalyst,Carrier and metal-The chemical nature of the support interactions (MSIs) strongly regulates the activity of the catalyst.CeO2It is a key component of automotive catalytic converters, especially with the strength of precious metals.MSIsIt is known for keeping them stable in highly dispersed form. To limit precious metalsThe agglomeration of the active phase in SACs, usually using low metal loading, high surface area support, and nanostructures can significantly change the CeO2The chemical nature.but currentlyCeO2The effect of particle size on the catalytic performance of atomically dispersed noble metals has not been investigated.
Solution ideas
Based on this,Eindhoven University of Technology in the NetherlandsEmiel J. M. Hensen团队Useflame spray pyrolysis(FSP) Pd-CeO with variable carrier size (4~18 nm) were prepared in one step2SACs. UseCO oxidation was used as a probe reaction to demonstrate the preparation of nanocompositesThe reaction activity is strongly dependent onCeO2The size of the particles. By combining advanced in-situ spectroscopy tools and steady-state kinetic studies,Pd-CeO2The size-dependent redox characteristics of the interface have a strong influence on the catalytic performance.The relevant results are based on 《Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts》为题发表在ScienceOn.

different sizesCeO2Supported palladium catalyst
Using a solution containing two metal precursors, in one step1wt % Pd/CeO prepared by FSP process2Catalyst(PdFSP)。HAADF-STEM图像(Figure1A) shows the synthesis of Pd/CeO2is octahedronPdFSP catalyst:(I) small ~ 4 nm NPs, (ii) medium ~ 8 nm NPs, (iii) large ~ 13 nm NPs.XRD only shows CeO2reflection, indicating that there is noPd/PdO相(Figure1B)。H2-TPR determinationPd-CeO in PdFSP nanocomposites2The interactionCeO2Strong influence of size(Figure1C). The first reduction peak (α) of small PdFSP NPs at ~ 200°C indicates that CeO2andThere is a strong interaction between Pd.WithThe increase in the size of PdFSP NPs, the feature shifts to low temperature, and finally splits into two peaks, indicating that the MSIs effect is weak.XPS results show that Pd in medium and small PdFSP samples (Figure2A) in highly dispersed Pd2 morphological existence, andCeO2strong interaction. For largerPdFSP NPs (>10 nm), an additional weak component was observed at ~ 336 eV, attributed to PdOxClusters.EXAFS shows that the Pd-oxo of all samples are mainly isolated, with about 4 O atoms in the first coordination shell and 2 to 3 Ce atoms in the second coordination shell (Figure2B). In the EXAFS of large PdFSP NPs, the weak contribution of Pd-Pd scattering at ~ 2.7 Å and coordination number ~ 1 indicates the presence of small PdOx clusters.In order to determineMorphology of Pd on the surface of NPs, DRIFTS adsorbed at -20°C using CO(Figure2C). In-situ drift spectra of small and medium-sized PdFSP NPs show that at ~ 2140 cm− 1There is a major infrared band, is the oxidation of single atomsLinear adsorption of CO by Pd species. For larger PdFSP NPs, at lower frequencies (2096, 2050 and 1900 cm− 1The additional CO band observed under) may be related to the presence of semi-reduced and aggregated Pd species.Palladium in largeCeO2NPs surface enrichment, thereby reducing the dispersion of palladium.
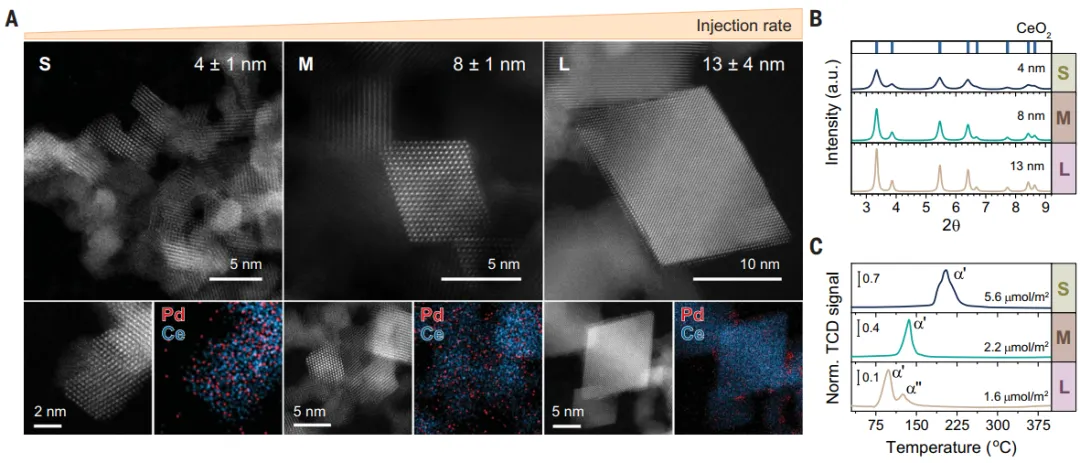
Figure 1: Preparation of different sizes of CeO by FSP method2carrier1wt% Pd-CeO2Structure and Reduction Properties of Nanocomposites
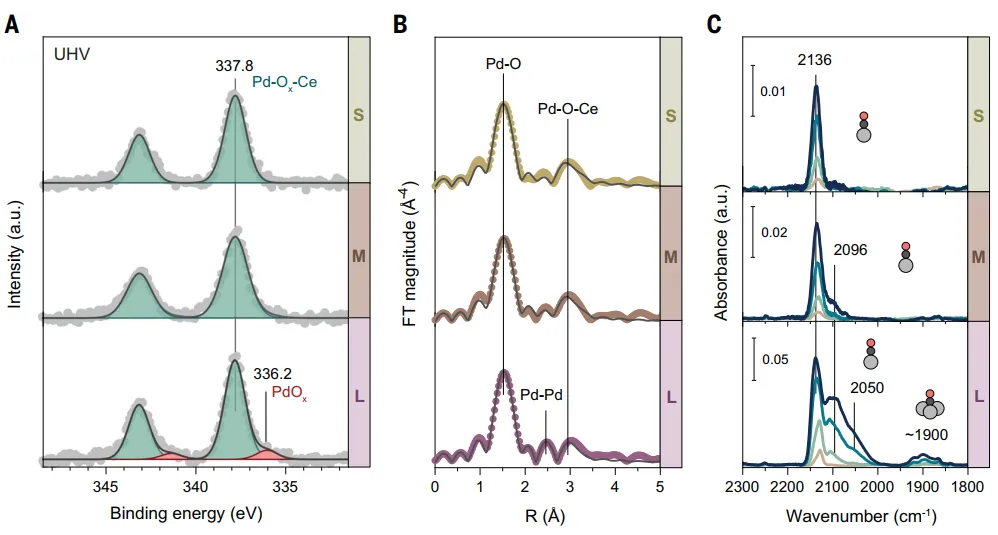
Figure 2: small, medium and large CeO2NanoparticlesSpectral characterization of PdFSP samples
CatalysisFormation of palladium during CO oxidation
used for researchDetermination of Pd/CeO Prepared by FSP by CO Oxidation2activity of nanocomposites.AllPdFSP catalysts can oxidize CO (Figure3A)。low temperatureCO oxidation activity (<100 ℃) and CeO2The size of the carrier is closely related. at moderate temperature(~ 150°C), the activity of small PdFSP NPs was lower than that of medium and large PdFSP NPs. According to the ignition catalytic test,~8 nm的CeO2NPs are best suited for low-temperature CO oxidation on PdFSP NPs(Figure3B)。Using in situ drift to reveal low temperatureThe morphology of Pd under CO oxidation conditions (75°C). Figure 3Cdisplay: even inAfter the reaction at 300°C, the Pd single atom is still dominant in the small PdFSP NPs.the dispersion of atoms in a medium-sized samplePd concentration is higher. On large PdFSP NPs, the proportion of metal and cluster palladium is significantly higher than that of atomically dispersed palladium, which is the reason for its limited low-temperature activity.In order to determinePd-oxo the stability at high temperature (300°C), a near-ambient pressure XPS(NAP-XPS) based on a surface-sensitive synchrotron is used. at high temperatureDuring CO oxidation, palladium is stable in the form of highly dispersed Pd-ox-Ce groups (Figure3D). The drift of medium and large PdFSP NPs shows that,Reduced formation at low temperaturesPd clusters, the formation of reduced Pd clusters after heating to 300 ℃ is more obvious.SmallSintering Resistance of Monoatomic Palladium in PdFSP NPs Derived from Strong MSI.WithCeO2As the size increases, these interactions become weaker, resulting in atomic dispersion under reaction conditions.The stability of Pd decreases. The observed CeO2The carrier size effect may be derived from the redox properties ando differences in mobility.
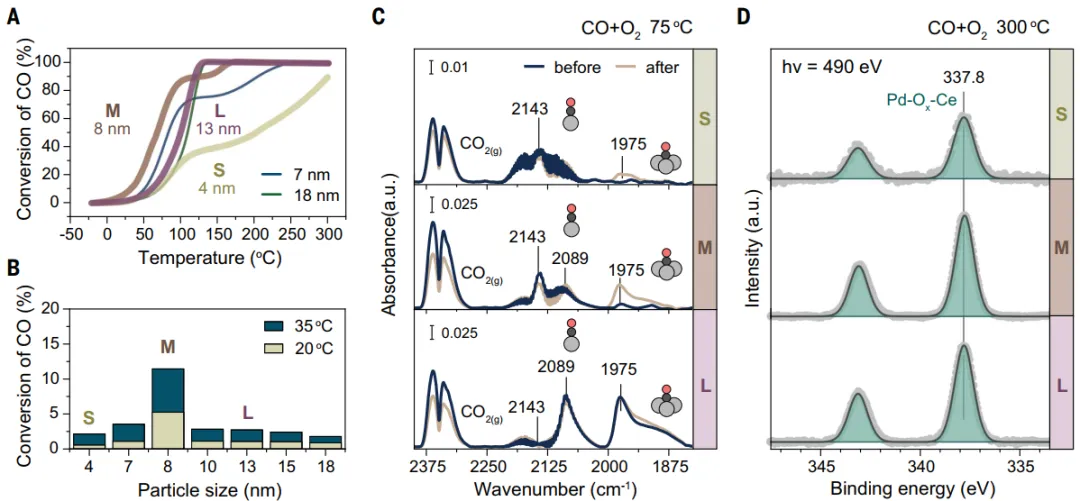
Figure 3: Catalyst performance and Pd morphology under reaction conditions
Pd-CeO2oxygen mobility at the interface
In situ resonance photoelectron spectroscopy(RPES) for the preparation of nanocomposites in Pd-CeO2The redox characteristics of the interface were studied.SmallValence band photoelectron spectra of PdFSP NPs (Figure4A) shows Ce3 Resonant peak relativeCe4 peak and the relative intensity of the non-resonance spectrum is the highestWhat, what?3 with the contentCeO2The reduction of NPs size decreases, indicating that the reducibility of the surface is related to size.size-inducedChanges in the redox properties of PdFSP NPs strongly alter the kinetics of CO oxidation. For~ 4 NM admin2The catalyst,The reaction order in CO is particularly high (1.3), while for larger NPs (>7 nm), the reaction order is greatly reduced, and the PdFSP NPs at 13 nm are 0.2 (Figure4B). this shows that,CO Oxidation on Large PdFSP NPs May Follow Mars-van Krevelen Mechanism. in the smallThe unusual sequence of reactions observed in PdFSP NPs suggests that O2Poisoned with smallCeO2The Pd site in the catalyst of NPs requires high pressure CO to obtain high catalytic activity. Under CO-rich reaction conditions, small PdFSP NPs showed higher activity than large PdFSP NPs (Figure4C)。
In order to directly determineEvolution of CO coverage on Pd sites, in situ DRIFTS experiments were performed under conditions similar to those used in the reaction sequence studies (Figure 4D). For largerPdFSP NPs, higher CO partial pressure caused only Pd speciation and CO2small changes in the intensity of the infrared band, reflectingOxidation activity of CO. For medium PdFSP NPs, the addition of more CO increased the activity, but also caused the decrease and aggregation of Pd-oxo species.Through in situ Raman spectroscopy and isotope labeling experiments, it is further proved that it has a small.CeO2The PdFSP catalyst of NPs has high O mobility. Pd induced O during low-temperature CO oxidationvformed and promotedO2of activation.
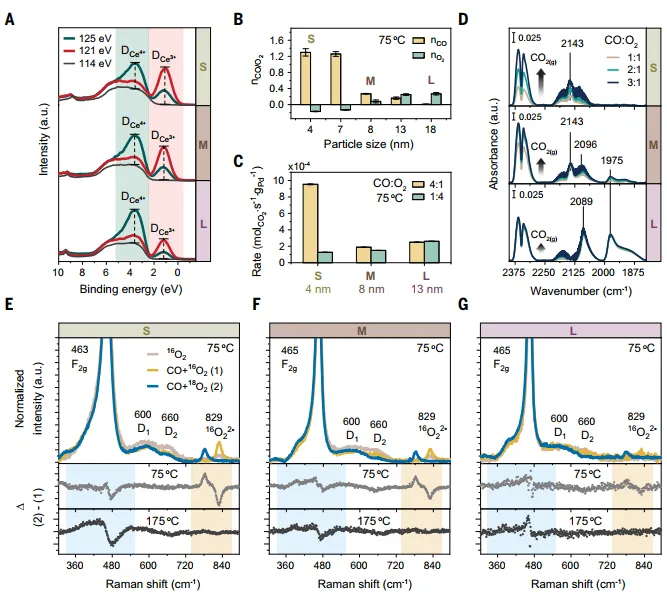
Figure 4:Pd-CeO2InterfaceCO Oxidation Kinetics and O Transfer
Summary
CeO2The size of the carrier can strongly affect the highly dispersedPd-CeO2Catalyst PairReactivity of CO oxidation.Pd和CeO2between the strongHigh O Mobility of MSI and Small PdFSP NPs (4nm) Lead to Pd Monoatomic O2Poisoning, limiting activity. MediumPdFSP NPs (8 nm)中CeO2The moderate reducibility of NPs and the mobility of O at the metal-support interface lead to a lower reaction sequence of CO and an earlier ignition time of CO oxidation. Due to weak MSI and weak O transfer, single Pd atoms are easily reduced and sintered in large PdFSP NPs (13 nm), which leads to poor low-temperature activity of the catalyst.Metal-CeO2The concept that the redox properties of the interface are dependent on the carrier size can be extended to otherCe3 /Ce4 Kinetically important catalytic reactions, suchCO2Hydrogenation, water-gas shift reaction and propane dehydrogenation.
References:
https://www.science.org/doi/10.1126/science.adf9082
Valery Muravev et al.Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts.Science (2023).
DOI:10.1126/science.adf9082

Artikel sebelumnya
Informasi terkait

Hubungi kami
Situs web: Website
Alamat: No. 66 jalan selatan Xinyuan, distrik Haicang, Kota Xiamen, Provinsi Fujian, Tiongkok

Kode QR akun resmi






