New breakthrough in chlor-alkali industry: organic catalyst, is expected to significantly reduce energy consumption!
2024-08-02
In the chlor-alkali process, since the nineteenth century, the electrolysis of sodium chloride solutions producess

In the chlor-alkali process, since the nineteenth century, the electrolysis of sodium chloride solutions produces chlorine and sodium hydroxide, both of which are important to the chemical manufacturing industry. Since the process is a high energy-consuming process, 4% of the global (about 150 TWh) electricity will be used in the chlor-alkali industry, and even modest efficiency improvements can bring considerable cost and energy savings. A particular concern in this regard is the harshchlorine evolution reaction.
Key issues
However, the chlorine evolution reaction still has the following problems:
1. Existing electrocatalysts are still developed decades ago
The most advanced electrocatalysts for chlorine evolution are still the dimensionally stable anodes developed decades ago. Although new catalysts have been reported, they are still dominated by precious metals.
2, non-precious metal catalyst efficiency is still not up to expectations.
Throughout the history of the chlor-alkali industry, many chemists have worked to replace noble metal catalysts with non-metal catalysts, however, non-metal catalysts have never achieved the desired efficiency.
Organic catalysts are rarely explored in electrochemical environments
Organocatalysts have long played an important role in organic synthesis and offer the advantage of being synthetically accessible and not limited by the availability of raw materials, but have rarely been explored in an electrochemical environment.
new ideas
in view of this,Academician Li Yadong and Wang Dingsheng of Tsinghua Universityand others foundOrganic catalysts with amide functional groups enable chlorine evolution reactions, and inCO2existence, it achieves10 knots2The current density andThe selectivity is 99.6%, and the overpotential is only 89mV, so it is comparable to a dimensionally stable anode. The author found that,CO2Reversible binding to amide nitrogen promotes the formation of free radical species, while free radical species inCl2plays a key role in the generationCl-Battery and organic synthesis aspects may also be useful. Although organocatalysts are generally considered to have no future in demanding electrochemical applications, this work demonstrates their broader potential and the opportunities they provide for the development of industrially relevant new processes and the exploration of new electrochemical mechanisms.

Technical scheme:
1. Clarified the chlorine precipitation process and electrochemical characterization
The overall electrochemical characterization indicatesNCOOH shows comparable or even better performance than DSA. Considering its low cost, NCOOH is therefore expected to be used in the chlor-alkali industry.
2. Analyze the reaction mechanism of chlorine precipitation
The authors used in situ attenuationNCOOH performed total reflection surface enhanced infrared absorption spectroscopy (ATR-SEIRAS), indicating the formation of N-Cl and N-COOH, which is attributed to CO2,Reaction between NaCl and RCON-H.
3. The structure-activity relationship of organic catalysts was clarified
Through theoretical analysis, the author believes that the nitrogen radical intermediate.1a-Int-5-pIt plays a key role in catalysis and is the reason for high catalytic efficiency.
Technical advantages:
1. For the first time, it was found that organic molecules containing amide groups can catalyze chlorine precipitation.
The authors found that organic molecules containing amide groups are able to generate chlorine, whereasCO2The addition of produces an intermediateNCOOH, which can exponentially increase the catalytic efficiency.
2. Achieved catalytic efficiency comparable to metal catalysts
The authors used organic molecular catalysts to achieve10 knots2The current density andThe selectivity is 99.6%, the overpotential is only 89mV, and the catalytic efficiency is comparable to that of the dimensionally stable anode.
3, can achieve CO2andCl2Direct separation
After the reaction is over, depending on the difference in the physical properties of the gas, you can directlyCO2withCl2Separation, recycling and reuse. AlthoughCO2The membrane permeates through the separation of the cathode and anode chambers, but the rate is too low to have a significant impact on the cathode process.
Technical Details
Chlorine evolution reaction and electrochemical characterization
The authors show that organic molecules containing amide groups catalyze the chlorine evolution reaction andCO2andCl2The separation and recycling process. The three-electrode system test shows that under industrial operating conditions,NCOOH at 10kA m2when it hasSmall overpotential of 89mV. In the absence of iR correction, NCOOH exhibits similar efficiency to DSA, which may be due to the relatively high resistance of organic molecules. In a practically more relevant two-electrode system, NCOOH also performs well and can reduce power consumption. At actual productivity,Production per ton using organic catalystsThe power consumption of NaOH is 1337 kWh, saving 4.6%. In addition, the electrochemical results show thatFast kinetics and small charge transfer resistance of the NCOOH system. NCOOH to Cl2Show moreHigh catalytic selectivity of 99.6%, and fairly stable performance.
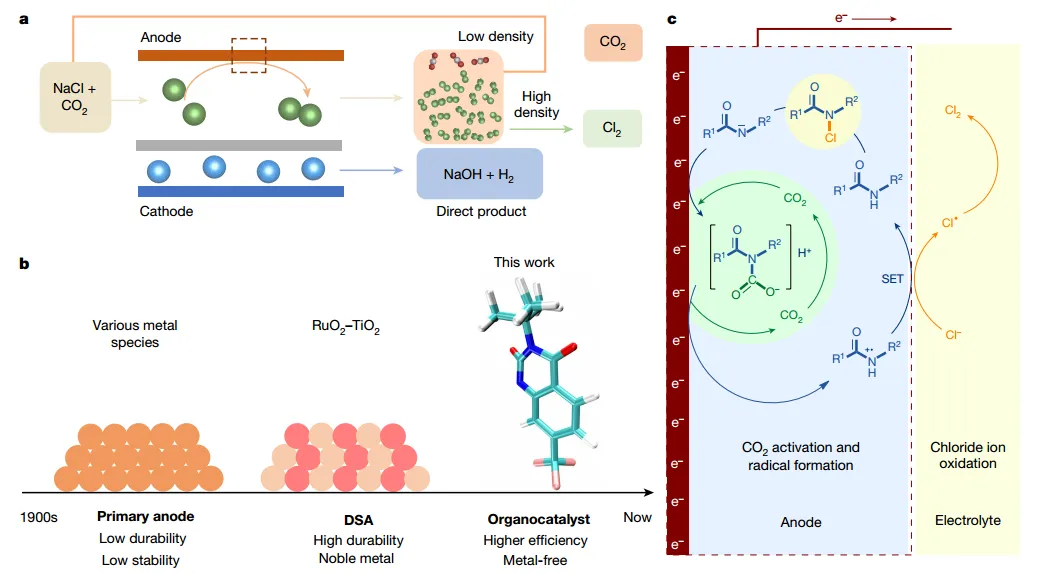
Figure1 Schematic Diagram of Chlorine Gas Production
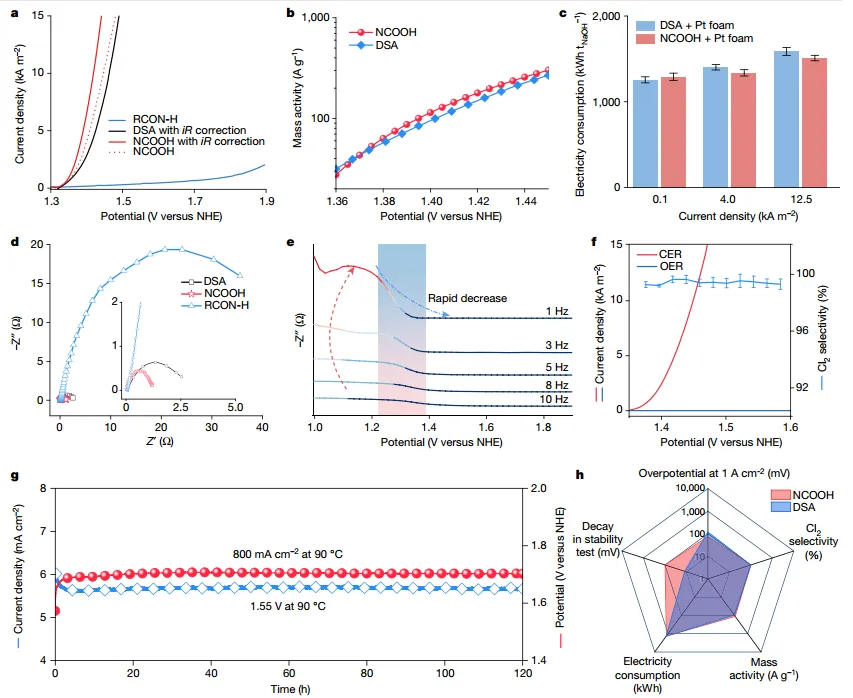
Figure2 Electrochemical characterization of CER
chlorine evolution reaction mechanism
In order to clarifyCO2The role of in situ attenuation onNCOOH was subjected to total reflection surface enhanced infrared absorption spectroscopy (ATR-SEIRAS). Observations suggest N-Cl and N-COOH formation due to CO2,Reaction between NaCl and RCON-H. Here the H atom on the amide group is first replaced by the Cl atom (1a-Int-1), and then the polarizedN-Cl bond and Cl-The reaction releases aCl2Molecules. generated in the processN-Intermediate (1a-Int-2) subsequently with CO2andH+rapid reaction formationN-COOH substances (1a-Int-4). Based on the above results, the authors elucidated the mechanism of CER reaction. When CO2When introduced into the system, the conjugate base(1a-Int-2) It is easy to capture CO on the anode2molecules and are able to form intermediates with lower redox potentialsNCOOH(1a-Int-4). For1a-Int-4,Cl−It is easy to be in the anode (1a-Int-5) is formed by oxidation by single electron transferCl •, can eventually form Cl2.Cl2After detachment, release1ainto the next catalytic cycle.
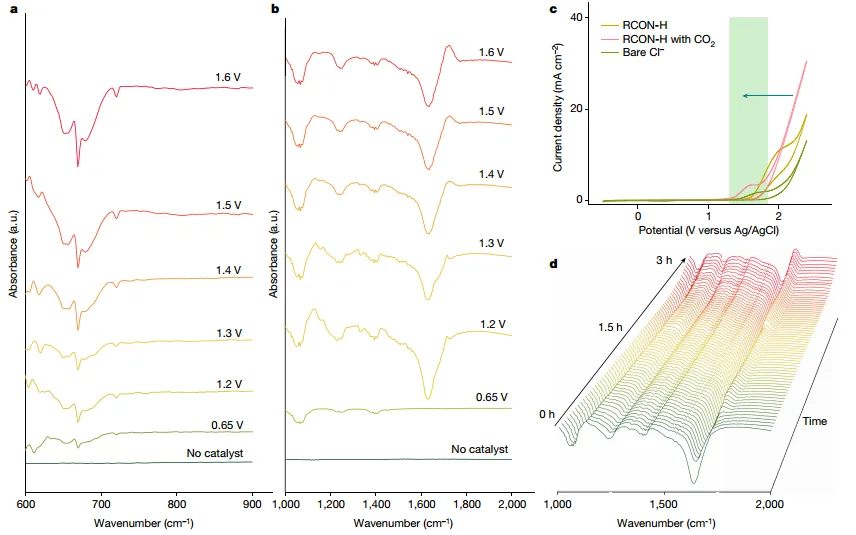
Figure3 ExploreCER mechanism
Structure-activity relationship of organic catalysts
Nitrogen radical intermediates before protonation (1a-Int-5-p) plays a key role in the reaction, so further theoretical analysis is needed. The author provides1a-Int-5-pdifferent visualizations of electronic states,1a-Int-5-pThe value of the electrostatic potential is lower than the surrounding atoms, which will replenish the chloride ions and provide active sites.1a-Int-5-pThe electron localization energy level is low, and the electron localization function value is lower.0.5, indicating1a-Int-5-pThe electrons are ion-like and may beCER active site. The authors note that functional groups are1a-Int-5-pThe upper spacing is close, which may indicate a synergistic effect. however,1a-Int-5-pThe Laplace analysis of the electron density reveals a clear defect in favor of electron transfer from the direction indicated by the arrow, and shows that1a-Int-5-pis the cause of the high catalytic efficiency seen.
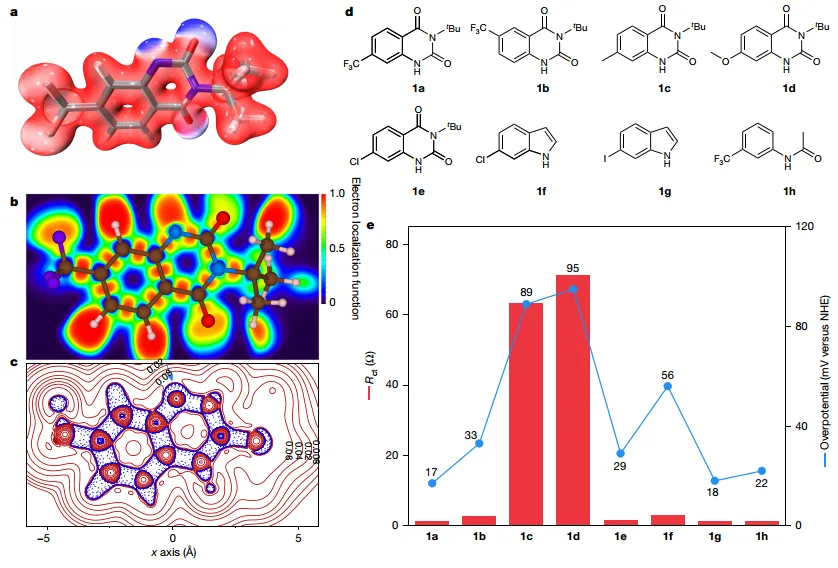
Figure4 Structure-activity correlation of organic catalysts for CER
Outlook
Anyway,The author found that organic catalysts with amide functional groups can achieve chlorine evolution reaction, and inCO2catalytic performance comparable to stable anodes can be achieved in the presence.Cl radicals and amide radicals formed during CER may be beneficial not only to the chlor-alkali industry but also to organic synthesis. And the demonstrated CER activity and selectivity are comparable to DSA, suggesting that organocatalysts are more promising in demanding electrochemical applications than commonly believed. Clearly, there are many opportunities to explore at the intersection of organic chemistry, inorganic chemistry, and electrochemistry.
References:
Yang, J., Li, WH., Tang, HT.Et al. CO2-mediated organocatalytic chlorine evolution under industrial conditions. Nature 617, 519-523 (2023).
DOI: 10.1038/s41586-023-05886-z
https://doi.org/10.1038/s41586-023-05886-z

Artikel sebelumnya
Informasi terkait

Hubungi kami
Situs web: Website
Alamat: No. 66 jalan selatan Xinyuan, distrik Haicang, Kota Xiamen, Provinsi Fujian, Tiongkok

Kode QR akun resmi






