This Science, re-examine the catalyst reconstruction!
2024-08-02
In this paper, by the study of foreign exchange technology The original writing of the center aims to share relevant

Special Notes:In this paper, by the study of foreign exchange technology The original writing of the center aims to share relevant scientific research knowledge. Due to limited knowledge, there are inevitably omissions and mistakes. Readers are requested to read critically and to criticize and correct them.OriginalTong heart is not lost(Xue Yan Hui Technical Center) EditorFengyun
research background
Supported metal catalysts respond to pretreatment and reaction conditions by recombination, phase changes, and chemical and structural oscillations, which can lead to catalyst activation, deactivation, or changes in selectivity. A good example of catalyst restructuring is the so-calledstrong metal-Carrier Interaction (SMSI), I .e. metal nanoparticles(NPs) are reduced or oxidized at high temperature by reducible carriers (such as TiO2) of the covering wrap. SMSI can lead to a large change in product selectivity, which is in line with the high activity of mixed metal oxides in the oxygen evolution reaction and industrial Cu/ZnOx/Al2O3The activation of the catalyst.
Key issues
However, the research on the reconstitution process of the catalyst in the reaction process still has the following problems:
1. Little is known about the structure of the cover layer under reaction conditions.Although there is substantial evidence of overburden formation during catalyst pretreatment, including fromH2,O2andCO2Atomic resolution in situ electron microscopy studies of the formation of the capping layer under ambient conditions, but little is known about the structure of the capping layer under reaction conditions.
2. The effect of the covering layer on the catalytic performance under the reaction conditions is still unknown.Recently, in situTEM shows Pt/TiO2Completely removedTiOxoverburden, but whether the overburden persists under the reaction conditions, or is used to induceIt remains unclear whether pretreatment of SMSI only indirectly affects catalyst performance.
3, the development of metal NP requires a multi-scale operation method.Multi-scale operation methods (from individual nanoparticles to the overall level) are useful for supporting metals. It is essential for NPs to develop meaningful structure-performance relationships.
new ideas
in view of this,Utrecht University, The NetherlandsBert M. Weckhuysenby a combination of in situ electron microscopy and vibrational spectroscopy, et al.Thin TiO formed on a nickel/titania catalyst during reduction at 400°CxThe capping layer was completely removed under carbon dioxide hydrogenation conditions. On the contrary, inAfter reduction at 600°C, exposure to carbon dioxide hydrogenation reaction conditions resulted in only partial re-exposure of nickel to form the same TiOxinterface sites of contact, and by providing a pool of carbon species in favor of carbon-Carbon coupling. The findings of this workChallenged yes.traditional understanding of SMSI and calls for more detailed operational studies of nanocatalysts at the single-particle levelto re-examine the structure-Static model of liveness relations.

Technical scheme:
1. The partial coverage of Ni in the reduction process at 400°C was analyzed.By means of in situ electron microscopy experiments, the authors investigated the loading at the single-particle level The formation of TiOx coating on Ni NPs, the surface selective coverage of Ni NPs is explained by theoretical calculation.
2. Ni/TiO is shown2InCO2in the hydrogenation processNi re-exposureThe author followed.CO2hydrogenation reaction conditionsTiOxThe change of the overlayer confirms the dynamic evolution of the catalyst during the electron microscope observation to express its more active surface.
3. The reduction of Ni/TiO at 600°C was explored2ofTiOxOverburden formation and reorganizationThe author prepared600-Ni/TiO2, showing a fully encapsulated and relatively thickTiOxoverlay, inCO2under the hydrogenation reaction conditions,600-Ni/TiO2are still mainlyTiOxThe package.
4. The catalytic performance was compared and the reaction mechanism was analyzed.Catalytic tests showed that both catalysts wereCO at 200°C to 400°C and 5 bar2Hydrogenation is active,600-Ni/TiO2C of the catalyst2 Selective enhancement becomes more pronounced. proposed a recombination under reaction conditionsNi/TiO2model of the catalyst to explain the variation in catalyst performance.
Technical advantages:
1. For the first time, the recombination of metal oxide coatings under operating conditions has been directly observed.For the first time, the reorganization of metal oxide coatings under operating conditions has been directly observed, and their effects on the reduction temperature have been rationalized.CO/CO2in hydrogenation reactionEffect of Ni activity and selectivity.
2, reveals the reaction process of Ni/TiO2On catalystTiOxEvolution of overburdenThe authors reveal that at low temperatures(400°C) and high temperature (600°C) after reduction, used for CO and CO2Hydrogenation reactions of industrial relevance in catalytic processesNi/TiO2On catalystTiOxEvolution of the overlay.
Uncover information at the single-atom level with unprecedented resolutionCombining multiple characterizations and calculations to observe and understand superlayer reorganization and its impact on catalysis, the combination of nano-and bulk-operation analysis techniques reveals information at the single-atom level with unprecedented resolution.
4. Provides new insights into the properties of the loaded metal nanoparticle systemThe author customized the low-oxide coating to keep it stable under the reaction conditions of different reduction pretreatment temperatures. The results can help understand the performance of many supporting metals in sustainable technical reactions, such as biomass andCO2High-value utilization, etc.
Technical Details
Partial coverage of Ni during reduction at 400°CIn order to passSMSI-induced oxide coating and its application in CO2role in hydrogenation, the authors in 400°C (400-Ni/TiO2) reduced 6 wt% Ni/TiO2Catalyst. By in situ electron microscopy experiments in a window gas chamber,The loaded are studied at the single-particle level Ni NPs上TiOxFormation of cover layer. The author analyzedThe particle size of Ni NPs is also confirmed by the particle size of Ni NPs. by 400-Ni/TiO2in situHAADF-STEM imaging, TiO was detected on Ni NPsxThe cover layer corresponds to the partially encapsulated double layer. The author calculatedThe strain changes and Ti-Ti and Ni-Ni of the Ni surface atomic column further confirm the continuous TiOxOverlay. Through theoretical calculations, it is found that inFormation of TiO on Ni(111)2cover with a lower energy loss, explaining the experimentally observed The surface selective coverage of Ni NPs, and shows that the cover layer is likely to be a low oxide phase.
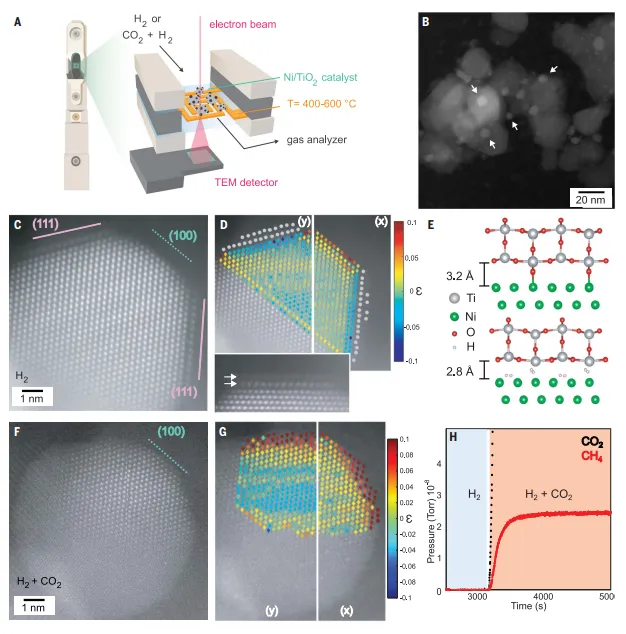
Figure 在Ni/TiO2Hydrogenation catalyst through low temperatureH2reduction formationTiOxOverburden and its presence inCO2RECONSTRUCTION IN THE PROCESS OF METHANATION
Ni/TiO after 400°C reduction2InCO2in the hydrogenation processNi re-exposureAuthors by usingHAADF-STEM track a single Ni NP while at 400°C and atmospheric pressure from H2SwitchCO2:H2, trackingCO2hydrogenation reaction conditionsTiOxOverlay changes. Mass spectrometry was used to track the reactant and product gases from the windowed gas chamber in real time during the reaction. IntroductionCO2:H2After the mixture can be detectedCH4The formation of the catalyst was confirmed during the electron microscope observation.CO2Hydrogenation.Ni NP formed a TiOxcapping layer, which, prior to hydrogenation, selectively occupies(111) surface of Ni. Exposure to CO2After the sameNi NPs reorganize and the NPs take on an overall rounded morphology and retain their Ni metallic phase. Supported Ni catalyst in CO/CO2The hydrogenation process evolves dynamically to express its more active surface. The author measuredDecay of the Ni 2p signal relative to the Ti 2p signal in NAP-XPS, indicating that exposure to CO2under hydrogenation conditionsTiOxThe removal of the cover represents the majority of the sample.
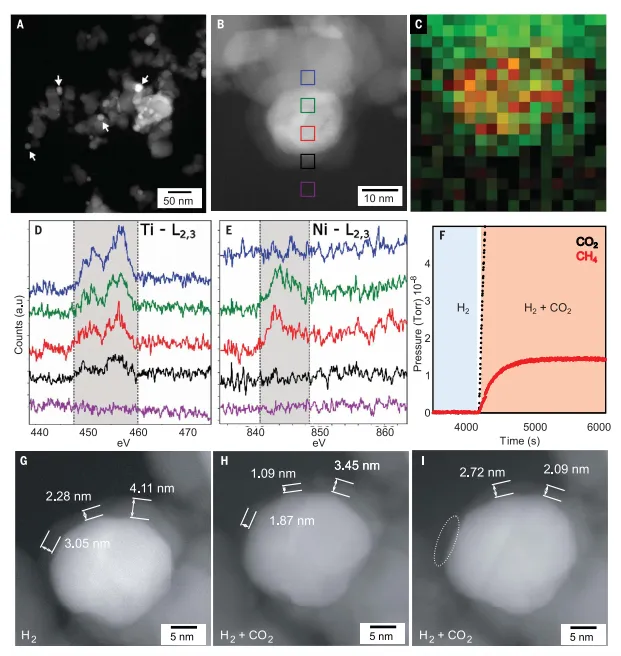
FigureReduction of Ni/TiO at high temperature2On hydrogenation catalystCO2stable during methanationTiOx/Formation of Ni coating
Reduction of Ni/TiO at 600°C2ofTiOxOverburden formation and reorganizationIn order to form a more stableTiOxoverlay, capableCO2survived under hydrogenation conditions, in 600°C(600-Ni/TiO2) in-situ reduction of 6 wt% Ni/TiO2Catalyst. itsThe NP diameter approximately doubled to 14.0 nm. 600-Ni/TiO2showed a fully encapsulated and relatively thickTiOxOverlay. Through in-situEELS analysis confirms that TiOxThe formation of the cover layer, which shows the entireTi L in Ni NP2,3Ionization edge signal. Compared to the original catalyst,TiOx-Low oxide overburden is still visible, but in CO2It became more inhomogeneous after exposure, indicating its partial degradation. In-situSTEM results show that in CO2under the hydrogenation reaction conditions,600-Ni/TiO2are still mainlyTiOxThe package. Therefore, it is expected that inAlmost complete loss of activity of such catalysts was observed in Ni-promoted reactions.
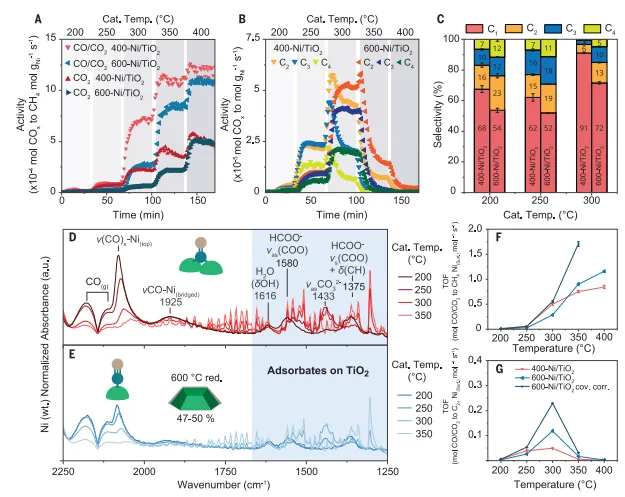
FigureIn situ vibrational spectroscopy reveals CO and2under hydrogenation conditionsNi/TiO2re-exposure
comparison of catalytic performanceThe catalyst was tested by in situ Fourier transform infrared spectroscopy under hydrogenation conditions, and the residualTiOxOverburden pair Ni/TiO2catalyst catalysisCO/CO2Effect of hydrogenation performance. The results show that the two catalysts inCO at 200°C to 400°C and 5 bar2Hydrogenation is active. In CO/CO2In the co-hydrogenation experiment,C of 600-Ni/TiO2 catalyst2 Selective enhancement becomes more pronounced. under the reaction conditions,600-Ni/TiO2The re-exposure of the Ni surface in the reaction affected the product selectivity.
mechanism analysis and structure sensitivityproposed a recombination under reaction conditionsNi/TiO2The model of the catalyst is used to explain the changes in surface chemistry and ultimately the changes in catalyst performance. In-situ The combination of FTIR spectra and HAADF-STEM results shows the formation of model structures. The authors propose that the increased Ni-TiOxinterface by providing an interface withThe adsorbate on Ni closely contacts the C species reservoir and assists in C- C coupling by stabilizing intermediate and transition states.
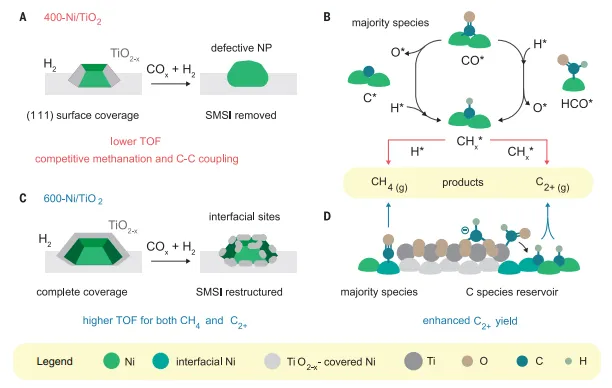
Figureon reduction and reaction process of Ni/TiO2New understanding of catalyst performance and evolution
OutlookIn short, the author directly observed the reorganization of metal oxide coatings under operating conditions for the first time, and rationalized their impact on the reduction temperatureCO/CO2in hydrogenation reactionEffect of Ni activity and selectivity. AuthorprovidedNi-TiOxOperational evidence for the formation of interfacial sites and demonstrate their effect on catalytic performance. Similar operating methods can be applied to understand many other chemical reactions. The key to this effort is to simultaneously measure catalytic performance and monitor and control the support, such as crystal phase, porosity, oxidation state and exposed surface, and supported active phase nanostructures, suchNP size, shape and composition.
References:MATTEO MONAI, et al. Restructuring of titanium oxide overlayers over nickel nanoparticles during catalysis. Science, 2023, 380(6645): 644-651DOI: 10.1126/science.adf6984https://www.science.org/doi/10.1126/science.adf6984

Informasi terkait

Hubungi kami
Situs web: Website
Alamat: No. 66 jalan selatan Xinyuan, distrik Haicang, Kota Xiamen, Provinsi Fujian, Tiongkok

Kode QR akun resmi






